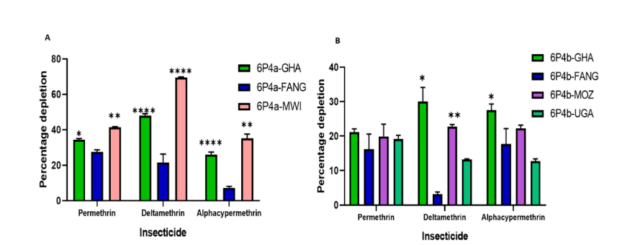
Illustration caption: Fig. 3 In vitro assessment of pyrethroid metabolism by CYP6P4a and CYP6P4b candidate alleles. A CYP6P4a recombinant enzyme metabolism of pyrethroids. B CYP6P4b recombinant enzyme metabolism of pyrethroids. Values are mean ± SEM of three experimental replicates compared with negative control without NADPH. Depletion by recombinant resistant enzymes is significantly different from depletion by FANG recombinant enzymes at *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001
This study combines computational analysis and functional genomics experiments to provide in-depth insights into the molecular mechanisms by which the duplicated CYP6P4a and CYP6P4b genes are driving pyrethroid resistance in Anopheles funestus. Genotype-phenotype association studies were also used to assess the impact of these genes on the effectiveness of current vector control tools.
Two key mutations were identified in the resistant mosquitoes: M220I in CYP6P4a and D284E in CYP6P4b. These mutations significantly increase the enzymes’ ability to metabolise pyrethroid insecticides and confer resistance to mosquitoes. In addition, transgenic expression experiments using Drosophila melanogaster flies revealed that the flies overexpressing the mutant variants of both genes were significantly more resistant to pyrethroids compared to the flies overexpressing the wild-type variants. These mutations were thus exploited in the development of two Deoxyribonucleic acid (DNA)-based resistance diagnostic tools (CYP6P4a-M2201 and CYP6P4b-D284E) to detect CYP6P4a – and CYP6P4b-mediated resistance in the field. With these tools, Nelly Tchatchoua and collaborators demonstrated using a genotype-phenotype approach that bearing these mutations is strongly linked to pyrethroid resistance and reduced efficacy of pyrethroid bed nets like Olyset®, PermaNet 2.0®, and DuraNet®.
Analysis of geographic distribution of these mutations revealed their high prevalence in sampled mosquito from Guinea, Ghana, Sierra Leone, and Benin, all West African countries. Routine use of these novel diagnostic assays will aid in rapidly detecting and monitoring pyrethroid resistance, supporting the implementation of effective resistance management strategies by National Malaria Control Programs. The diagnostic tools developed here are thus valuable additions to the insecticide resistance management resources available in Africa.
Read more: https://doi.org/10.1186/s12915-024-02081-y